[News Focus] Action plan to make Korea a global COVID-19 vaccine factory takes shape
By Shim Woo-hyunPublished : May 19, 2021 - 16:00
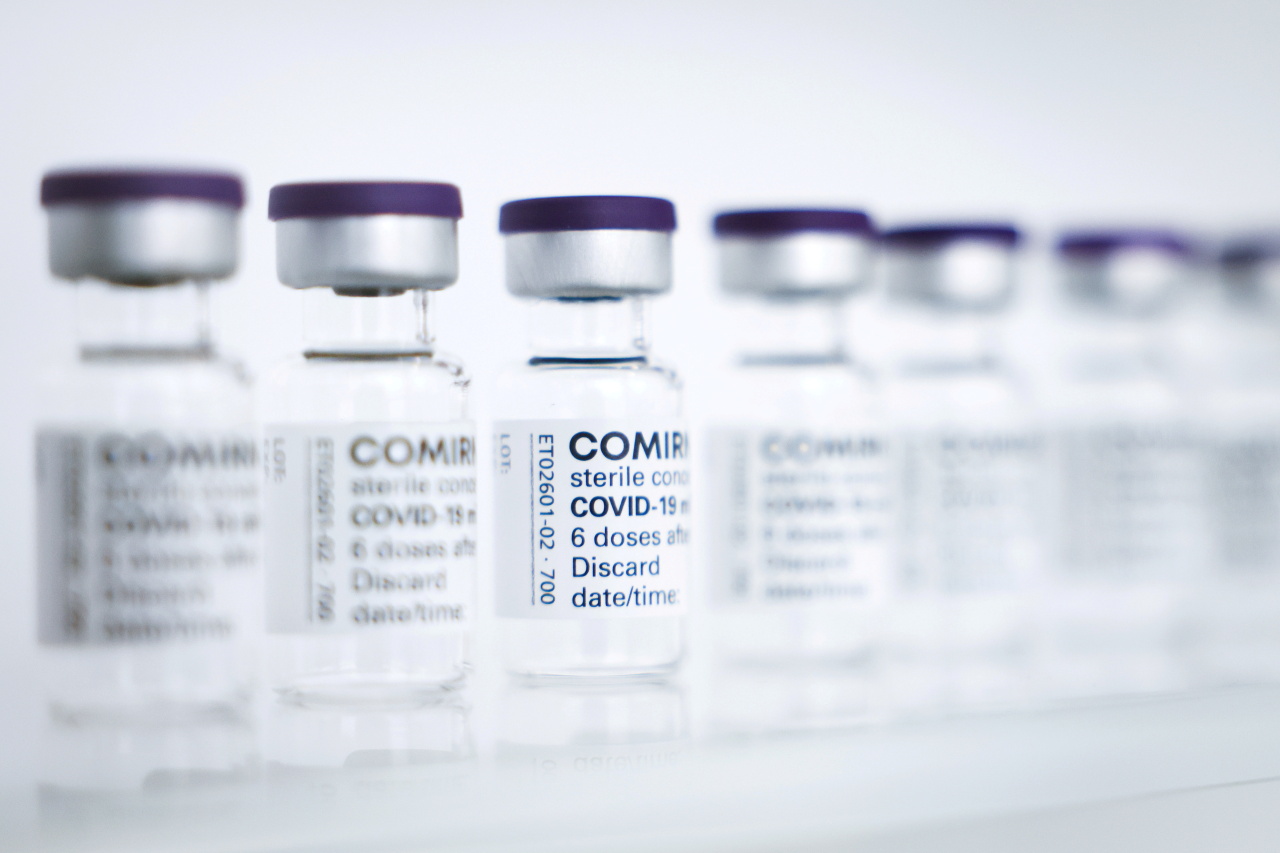
In the global fight against the COVID-19 pandemic, South Korea could step up as a global vaccine factory, if players unite to ramp up the country’s total production capacity, already the world’s second-largest, officials and industry insiders said Wednesday.
Talks have already begun among local biopharmaceuticals to forge a consortium for co-production of the doses -- in particular the entirely new type of mRNA vaccines developed by Pfizer-BioNTech and Moderna, the sources said.
The move is based on hopes of potential patent waivers and technology transfers related to US-developed vaccines, as state leaders of South Korea and the US are to meet for their first face-to-face meeting later this week. A vaccine partnership deal of some kind is on the agenda.
South Korean President Moon Jae-in, who departed for the US on Wednesday afternoon, made clear his intention to pursue a bilateral agreement on vaccines.
“I will make the upcoming summit an opportunity for advancing (the bilateral) cooperation on the vaccine front, and moreover, to position South Korea as a global hub of vaccine production,” Moon said.
Kurt Campbell, White House policy coordinator for the Indo-Pacific, further raised expectations on a Seoul-Washington vaccine deal.
“We are exploring a wide range of options to help increase vaccine manufacturing and distribution around the world,” Campbell said in an interview with Yonhap News Agency. “Both the US and South Korea are major vaccine manufacturers; we should work together toward bolstering the global supply of COVID-19 vaccines.”
Local bio industry officials are paying close attention to how discussions over possible intellectual property waivers for COVID-19 vaccines would develop.
US President Joe Biden previously proclaimed Washington’s support for the temporary suspension of intellectual property rights. Local experts and industry insiders cautiously hope that the US could choose to transfer the advanced mRNA vaccine technology to South Korea for it to mass manufacture the vaccines for global supply.
In that case, the envisioned consortium of local companies would combine the strength and capacity of all possible players to build a pan-industry system that can produce COVID-19 vaccines for both local and global consumption, industry sources said.
The companies that are part of this group currently include Hanmi Pharmaceutical, GC Pharma, GeneOne Life Science, ST Pharm, as well as institutions such as Seoul National University, Pohang University of Science and Technology and Myongji Medical Foundation.
The idea of an industry-wide coalition accelerated in April when the biotech firms were linked with Dr. Tom Frieden, former director of the US Centers for Disease Control and Prevention during the Obama administration, according to a source familiar with the discussions on the consortium.
The best scenario for South Korea to step up as a global production hub for COVID-19 vaccines is that the US government and US-based vaccine makers come to an agreement to waive patents and transfer related technologies for a certain price, the source said.
The consortium, in that case, could start producing the jabs in less than six months at existing facilities capable of producing 1 billion doses per year, according to the source. This current production capacity is largely from Hanmi Pharmaceuticals, a source noted.
With a joint effort, the production capacity would rise fivefold to supply 5 billion doses of vaccine within 12 months, the source said.
But Hanmi’s capacity is mainly in the stages of synthesizing mRNA and other ingredient, rather than completed vaccines, which contain complicated nano particles made of lipids and mRNA.
mRNA is the most advanced vaccine technology and no South Korean firms have advanced their technology to such levels.
The discussion between the two state leaders on Friday and follow-up actions are therefore of great importance to this vision, the official said.
It remains unclear as to how the two countries would cooperate on the vaccine front, but experts say a “vaccine swap agreement” -- a loan deal to ease a temporary shortage of jabs here, or bilateral agreements on intellectual property waivers and technology transfers could be possible.
A contract manufacturing deal is widely expected to be announced before or after the Moon-Biden meet, involving the world’s leading contract manufacturer Samsung Biologics.
The South Korean company is said to be close to signing a contract to manufacture Moderna’s COVID-19 vaccine at its plants in Songdo, Incheon. The firm’s CEO John Rim reportedly left for the US on Wednesday.
The Samsung-Moderna deal, if realized, would be a positive boost for South Korea, as it would help alleviate a vaccine shortage here in the short term.
Yet, it falls short of catapulting the country into a global hub for COVID-19 vaccines in the long term, a person involved in the consortium claimed. Samsung BioLogics is not taking part in the consortium talks.
“Securing or developing the technologies related to mRNA vaccines ourselves will provide us the grounds for South Korea to step up as a global production hub for the jabs,” the source said.
Should there be no technology transfer, the consortium may have to spend over two years to develop its own vaccine and go through clinical trials.
“It is uncertain how long it would take if the consortium is to self-develop its own vaccine,” the source said.
When self-developing mRNA COVID-19 vaccines, local companies that are joining the consortium are expected to combine their existing technologies.
ST Pharm, one of the members of the consortium, has recently purchased a lipid nanoparticle drug delivery technology from Genevant Science, a vital component of the new mRNA COVID-19 vaccines. Hanmi is also capable of manufacturing enzymes for nucleotide, plasmid DNA and mRNA synthesis. GC Pharma has facilities in Ochang, North Chungcheong Province, where the company could fill and finish up to 1 billion mRNA vaccine doses a year.
The consortium is expected to ask the government to inject trillions of won for development of its own vaccine as well, just as Moderna was given research aid from the US government.
The consortium has recently shared with government officials that local companies would need a substantial amount of funding to develop a homegrown COVID-19 vaccine.
According to another source from a local pharmaceutical company, Moon has hinted that the government could make an unprecedented amount of investment for the development of COVID-19 vaccines during a speech he delivered last week to mark his fourth anniversary in office.
By Shim Woo-hyun (ws@heraldcorp.com)










![[Weekender] Korean psyche untangled: Musok](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/05/02/20240502050841_0.jpg&u=)






![[Eye Interview] 'If you live to 100, you might as well be happy,' says 88-year-old bestselling essayist](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/05/03/20240503050674_0.jpg&u=)
